|
Hard
Core Computer System Characteristics
The Computational Element
The established input devices have proven themselves to be functional when
used separately. The innovation of the AGRID device is that AGRID will
utilize newly created integrated software that will allow all of the
separate components to work together. This will help to eliminate the
current need for constant user intervention to maintain healthy
blood-glucose levels—which is one of our main goals. Our other
primary goal is to provide insulin-dependent diabetes with a device that is
as non-invasive as current technology will allow. In order to fulfill
these requirement we will need to design new software as well as an
integration device to run the software.
System Design/Development Configuration
The design of
this software is fairly straightforward. The integration software will
read in the previously defined inputs (see section 1). Using these
inputs the device will then determine which of 5 actions need to be
performed. The user’s blood glucose levels can either be within
range, high, too high, low, or too low. The functional diagram F.1
clearly illustrates the functionality of the AGRID device.
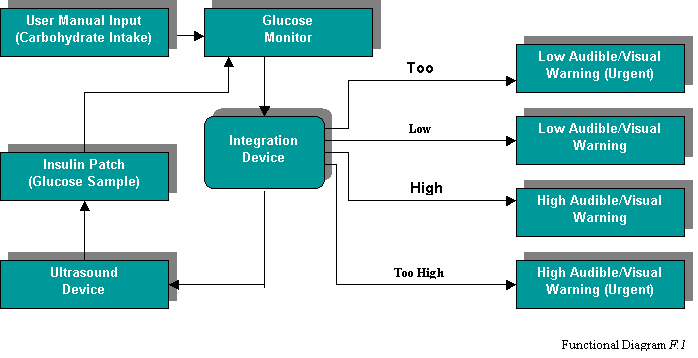
System
Integration Requirements
The
integration device must fulfill our established criteria. In order to
be considered successful the integration device must be able to read in the
inputs (see section 1). The integration device must come to a decision
about action based on the inputs received. Once the action has been
determined, the integration device will put the required action into motion
by sending information to the ultrasound device. The ultrasound device
will take the signal that it receives from the integration device. The
signal that the ultrasound component receives will determine the length of
time that the ultrasound device emits the ultrasound rays. The
duration of the ultrasound session directly determines the rate at which the
insulin is absorbed into the skin.
System Test Requirements
To gain FDA-approval the AGRID device must undergo stringent testing.
Before designing the prototype we will first test our integration device to
ensure its proper functionality. We will have our software tested
through an independent facility that will thoroughly test our integration
software for any errors or defects in the program. If there are
defects within our software, they will be resolved during this phase.
We will try to have all components issues resolved before we reach the
prototype development stage that corresponds with phase II of the SBIR.
Support Computer Based Application Programs
The Software Element
The
completion of this project requires the creation of new software that will
allow for the integration of the following components: glucose monitor,
insulin patch, and ultrasound device. These three devices are all
FDA-approved, currently available in the American market, and ready for use
in our project. Since all except for one of the components that we
plan to use are available off-the-shelf we feel that the integration of the
components will be successful.
Available
Off the Shelf Administrative Applications
AIDA Freeware
Available online at www.2aida.org there
exists freeware that may have some use in the creation of our software.
The AIDA freeware helps insulin-dependent diabetics adjust their insulin
dosage to better maintain their glucose levels. We will be able to use
this software to determine how much insulin should be dispensed in
consideration of the current glucose levels and the carbohydrate intake.
With this information we will create switch case statements that will
determine the actions taken by the integration device.
Applications Written for Project
If
the integration device determines that the blood glucose levels are within
the acceptable range then there will be no audible/visual warning and the
information will be simply be sent to the ultrasound device that will adjust
the skins level of permeability in taking into consideration the level of
insulin that is required to bring the wearer’s glucose level to the
optimum level of 100.
If
the glucose level/carbohydrates is either low/low or high/high an alarm
notify the wearer while information is sent to the ultrasound device.
This warning alarm will steadily flash the wearer’s glucose level as well
as produce an audible beep once every 5 seconds to notify the wearer.
The user can disengage this warning at any time with the push of a button
located on the user interface. If the wearer’s blood glucose levels
reach the too high or the too low state, information will still be sent to
the ultrasound device in an attempt to control the glucose levels. The
audible/visual warning will then enter the urgent state. In the urgent
state the flashing of the blood glucose levels and audible warning will
still be performed; however, the frequentness in which they are done will be
increased to once per second. If in this state it will be recommended
to the wearer that an injection of insulin be dispensed manually to quickly
get the blood glucose levels within the desired range. This user will
also be able to disengage this warning if desired. If there is no user
intervention then the warning alerts will shut off automatically once the
blood glucose levels fall with the acceptable range. In all cases the
integration device will notify the ultrasound component to make adjustments
to skins permeability.
The
established ranges of the AGRID devices are defined within table T.1.
These ranges have been chosen in accordance to what the American Diabetes
Association has determined to be acceptable glucose levels. The
average carbohydrate intake level that should be consumed by diabetics for
each meal has determined the carbohydrate intake ranges that have been
established in this table. The integration device will then perform
the explicit that corresponds with the inputted information.
|
|
Inputs
|
Outputs
|
|
Case
Number
|
Glucose
Level
|
Carbohydrates
|
Insulin
Level
|
Warning
State
|
|
1
|
Too
Low
|
--
|
Minimal
|
Low
Warning (Urgent)
|
|
2
|
Low
|
Low
|
Minimal
|
Low
Warning
|
|
3
|
Low
|
Normal
|
Minimal
|
Off
|
|
4
|
Low
|
High
|
Minimal
|
Off
|
|
5
|
Normal
|
Low
|
Minimal
|
Off
|
|
6
|
Normal
|
Normal
|
Continuous
|
Off
|
|
7
|
Normal
|
High
|
Continuous
|
Off
|
|
8
|
High
|
Low
|
Continuous
|
Off
|
|
9
|
High
|
Normal
|
Increase
|
Off
|
|
10
|
High
|
High
|
Increase
|
High
Warning
|
|
11
|
Too
High
|
--
|
Increase
|
High
Warning (Urgent)
|
|
Glucose
Level
|
|
Too
Low
|
<=
50
|
|
Low
|
51-80
|
|
Normal
|
81-130
|
|
High
|
131-300
|
|
Too
High
|
>
300
|
|
Carbohydrates
|
|
Low
|
<=
5
|
|
Normal
|
6-15
|
|
High
|
>
15
|
Insulin
Level 10/20/30 mg
|
|
Minimal
|
.2/.4/.6
mg/h
|
|
Continuous
|
.4/.8/1.2
mg/hr
|
|
Increase
|
Increased
Linearly
|
Unique
Produce’s Domain Specific Applications
The
AGRID device is designed for insulin-dependent diabetics. AGRID will
not be helpful to those diabetics who are reckless in the management of
their diabetes regimen. Our product will also not be beneficial to
those who would not benefit from the dispensing of insulin. The
benefits of using this device are that a normally invasive, painful process
will be made simple and non-intrusive.
|


